News
Know more about Ozone
Ozone has been present on Earth for as long as oxygen itself—approximately 500 million years. It has existed in our universe for billions of years. Whenever oxygen is exposed to an electric arc, such as a lightning strike, or a specific wavelength of ultraviolet light, like that from the sun, ozone (O3) is formed.
Ozone, often referred to as "activated oxygen" or "atomic oxygen," consists of three oxygen atoms, unlike the regular oxygen (O2) we breathe, which contains only two. Ozone is one of the most effective sterilizers in nature, capable of neutralizing bacteria, viruses, and odors. It naturally forms in the environment, particularly during thunderstorms when lightning strikes, and is also produced by the Sun’s ultraviolet rays.
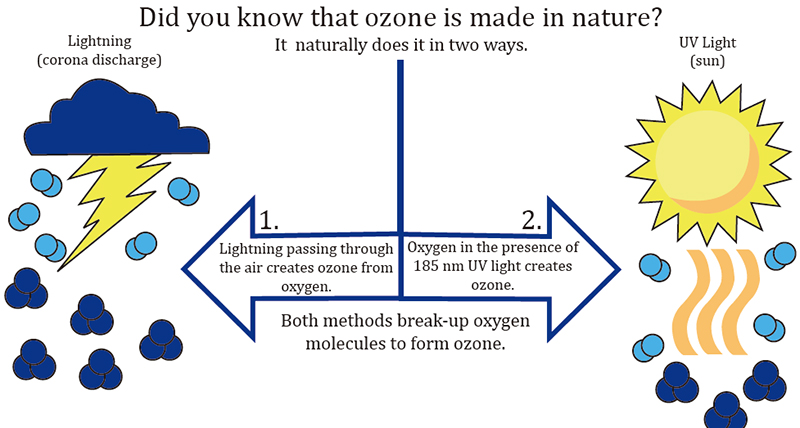
During an electrical storm, the intense energy from lightning strikes breaks apart oxygen molecules, leaving behind individual oxygen atoms. These single atoms quickly combine with surrounding O2 molecules to form O3, or ozone. The fresh, clean scent we often notice after a storm—also replicated in many laundry fabric softeners—primarily comes from this natural production of ozone. Lightning acts as nature’s air purifier.
Our awareness of ozone is largely tied to the "ozone layer" that surrounds the Earth’s atmosphere. The damage to this protective layer, caused by carbon emissions and pollution, has made headlines for over 35 years. The ozone layer, located in the lower stratosphere, is formed through molecular fission and fusion, primarily triggered by the sun’s short-wave ultraviolet rays, not electricity. Interestingly, this ozone layer, created by these short UV rays, shields life on Earth from the harmful longer UV rays in the spectrum.
Related News
- How Does Ozone Work ? 2019-09-10
- How Ozone Generators Work? 2020-01-08
 sales
sales