News
How Does Ozone Work ?
Generation: High-energy sources like ultraviolet (UV) light or electricity are used to strike the Oxygen (O₂) molecules.
Separation: The energy causes the O₂ molecule to split into two individual atoms, known as "activated oxygen" or "atomic oxygen."
Formation of Ozone: These atomic oxygen atoms then bond with other O₂ molecules, forming an O₃ (ozone) molecule.
Reactivity of Ozone: The newly formed O₃ molecule, with its third unstable oxygen atom, becomes highly reactive and electrically charged.
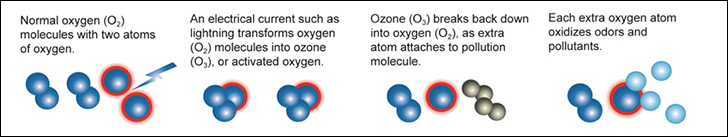
This reactive O₃ molecule readily attaches itself to other odor-causing molecules or contaminants. When it bonds with them, the unstable third oxygen atom detaches, and the ozone molecule reverts back to regular O₂ (oxygen). As it interacts with odors, bacteria, or viruses, ozone alters their chemical structure, turning them into less odorous or harmless compounds. This process is known as oxidation.
Ultimately, ozone breaks down back into oxygen after completing its task, making it an environmentally friendly and efficient oxidant that purifies the air without leaving harmful residues behind.
Related News
- Know more about Ozone 2019-03-05
- How Ozone Generators Work? 2020-01-08
 sales
sales